What is MAB Therapy for COVID-19?
FEB 16, 2022As we all are aware, the COVID-19 disease pandemic still exists today. Thankfully, we have monoclonal antibody therapy (MAB) as ...
Read More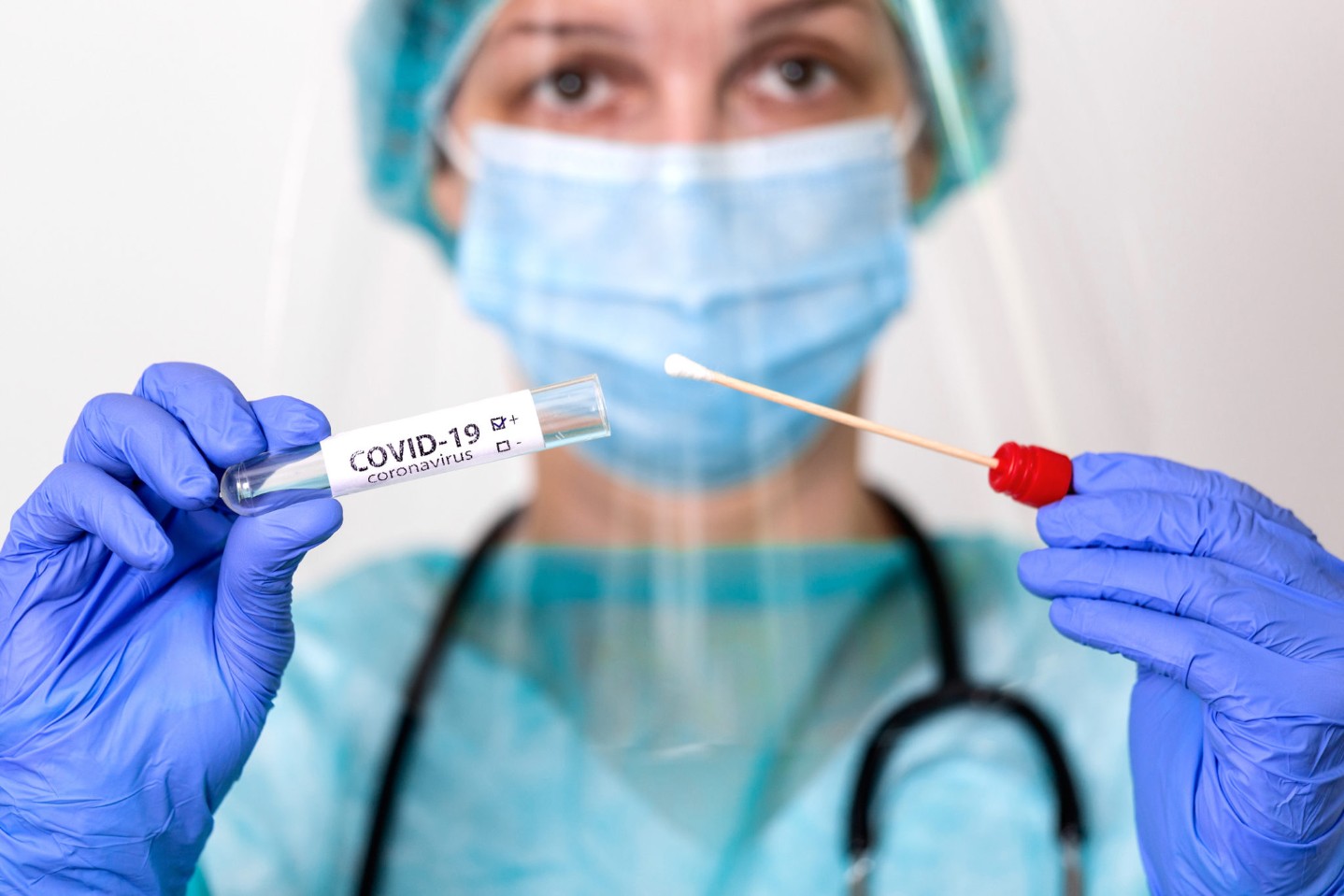
Since mid-March of this year, it is not an exaggeration to say that life has been turned upside down by something that is as old as time itself and microscopic in size. The newly discovered coronavirus (SARS-Cov-2 or HCoV-19) was first identified in the Wuhan, Hubei province in China, in December 2019, following cases of patients who developed pneumonia of unknown origin.
Coronaviruses themselves are not new and have been known to cause infection in the nose, sinuses and upper throat. Many infections that we refer to as the “common cold” are in fact caused by types of coronaviruses and are not dangerous for most people. However, SARS-Cov-2 has proven to be particularly troublesome causing severe illness and death.
There are several factors which serve to make this new coronavirus exceptionally concerning. First of all, since it is new, scientists and experts don’t have a complete understanding of it. Also it has proven to be very easily spread by person to person contact, and, finally, its ability to cause sickness is relatively unpredictable. In the eight plus months since the virus has been identified there have been some patterns develop that serve to predict risk. Those who seem to be at a higher risk for becoming ill are:
Social distancing, the use of face coverings, increased testing and quarantining have helped to mitigate some spread of the virus, but cases, illness and deaths continue to be problematic.
History has proven that the best way to combat viruses, especially those with the potential to spread rapidly and cause significant illness, is to develop a vaccine. Simply put, vaccines use various methods to mimic the virus that they protect against, which allows the body’s natural immune system to develop defenses specific to that virus. The good news is that in the United States, there are multiple entities working tirelessly on a vaccine for COVID-19 following the president’s Operation Warp Speed initiative. The government has thus far invested more than $10 billion to support this program. Several different vaccine production technologies have been proposed and studied in effort to hone in on the most effective and safest option.
The United States government’s Operation Warp Speed initiative has certainly served to accelerate the development of vaccines for Covid-19 (also known as SARS-CoV-2). There are more than 50 vaccine products currently in development worldwide with two distinct vaccines that are on track for FDA EUA (Emergency Use Authorization) within the next weeks. Products from Moderna and Pfizer are leading the way with the product from Pfizer / BioNTech receiving approval in the United Kingdom on December 2nd, 2020. The US FDA has scheduled a meeting of its Vaccines and Related Biological Products Advisory Committee for December 10th and December 17th to discuss the safety and effectiveness data provided by Pfizer and Moderna respectively for their Covid-19 vaccine products; with approval expected shortly thereafter. Data from both products shows effectiveness in excess of 90% against infection and almost zero against severe disease.
Some evidence suggests that individuals who have contracted Covid-19 may be susceptible to re-infection; meaning that the natural immunity developed from infection may not last. At this time it is not known whether a Covid-19 vaccine would be recommended for those who have previously developed an infection or have tested positive in the past.
At this point, there is still some conjecture among government officials about the groups of individuals who will be prioritized to receive the vaccine. It is clear however that front line healthcare workers and elderly persons (> 65years; especially those who are in long term care facilities and with multiple chronic conditions) will be among the first to receive the vaccine. This is likely to occur with the first month or two following approval and release of the vaccines. There are an estimated 21 million people working in health care and somewhere in the neighborhood of 3 million people in long term care facilities in the US.
Based on the known information at this time, estimates for when the vaccine may be available for the following phased groups are as follows.
For more questions around COVID-19, visit our resource area for Coronavirus.
Updated: Dec. 2020

As we all are aware, the COVID-19 disease pandemic still exists today. Thankfully, we have monoclonal antibody therapy (MAB) as ...
Read More
As COVID-19 evolves and persists in our communities, the conversation continues every day in homes, workplaces, and the media concerning ...
Read More
In January 2020, the World Health Organization announced that there was a mysterious Coronavirus- related illness that had first been ...
Read MoreWhen you need local health information from a trusted source, turn to the CHI Health Better You eNewsletter.